How FDA’s New Guidance on Digital Health Technologies (DHT) Impacts Staffing in Life Sciences

Ghosting: A Symptom of a Broken Hiring Process?
August 15, 2024
Cloud vs. On-Premise: Which Infrastructure is Best for Your Business?
October 11, 2024Digital health technologies (DHT) are transforming the way we think about healthcare and life sciences. Whether it’s wearable devices like fitness trackers or advanced software for remote patient monitoring, DHT is pushing the boundaries of patient care and clinical research.
The FDA’s new guidance, released in December 2023, introduces new responsibilities for life sciences companies, particularly around compliance and validation. These changes will directly impact how companies build and manage their teams to meet these new requirements successfully.
What is Digital Health Technology?
The FDA defines a digital health technology (DHT) as “a system that uses computing platforms, connectivity, software, and/or sensors, for healthcare and related uses.” These technologies offer many potential benefits, especially in clinical trials, patient monitoring, treatment development, and medical product development.
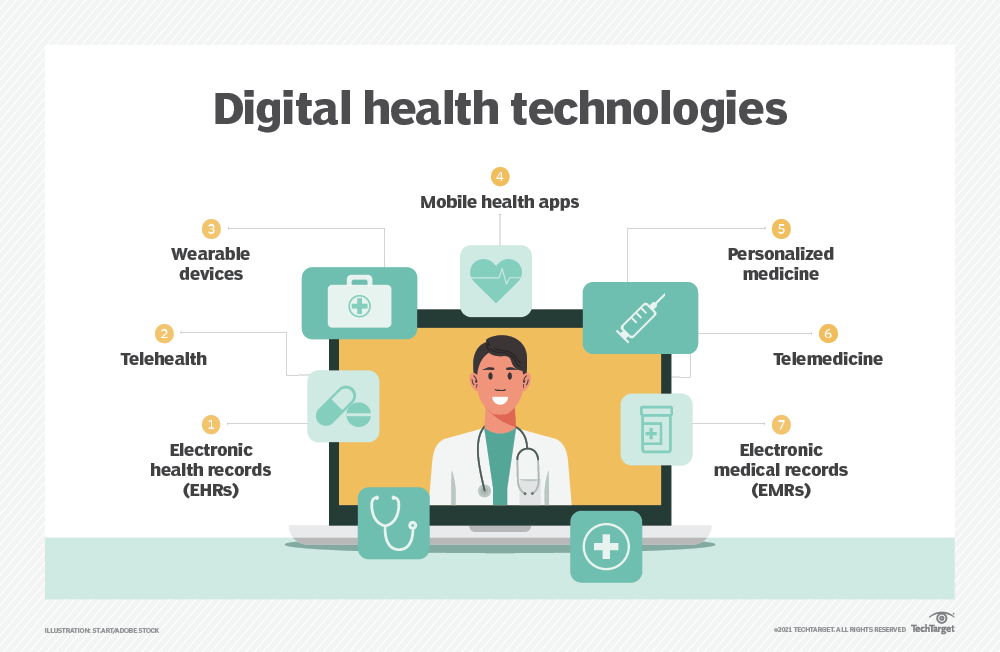
Digital health technologies | Source: TechTarget
A great use case can be seen in this recent study on respiratory diseases. It explores how home-based DHTs help monitor individuals with chronic respiratory conditions by identifying early changes in their health. This real-time monitoring allows healthcare providers to tailor treatment to each patient’s needs. It demonstrates the transformative potential of DHTs in improving care for people with chronic illnesses.
As the FDA issues new guidance for DHTs, life sciences companies must adapt to these guidance documents and related regulations. This includes a focus on validation and compliance. This is where the need for specialized staffing really begins to emerge.
How Does DHT Impact Staffing for Life Sciences?
As these technologies become more integrated into life sciences, companies will need to fill roles that combine technological expertise with regulatory knowledge. Here are a few areas where we see staffing needs evolving:
 1. Regulatory Compliance and Validation Experts
1. Regulatory Compliance and Validation Experts
With the FDA’s new guidance, life sciences companies must ensure their DHT solutions meet strict regulations. For example, in developing a smart insulin pump, validation experts test the device for accurate insulin delivery and reliable operation within defined parameters. They also verify that it adheres to FDA standards. These checks prevent potential issues and help to ensure patient safety.
2. Software and Data Engineers
Digital health tools are built on complex software and rely heavily on data. That means there’s a growing demand for software engineers and data specialists to develop, maintain, and secure these systems. They play a crucial role in making sure DHTs not only work as intended, but also comply with regulations like HIPAA for data protection.
3. Cybersecurity Specialists
With DHT relying heavily on connectivity, protecting patient data is a top priority. Cybersecurity specialists ensure that these systems comply with FDA and HIPAA regulations while safeguarding sensitive health information. Furthermore, a study from the National Library of Medicine highlights the increasing security threats posed by DHTs, including hacking, phishing, and malware. These vulnerabilities can compromise both patient privacy and system reliability. This emphasizes the need for robust security protocols like encryption, multi-factor authentication, and regular security training to mitigate risks and maintain trust in digital health systems.
4. Quality and Validation Specialists
Quality and validation professionals ensure that DHTs function correctly and meet quality standards. For instance, a pharmaceutical company is developing an automated drug dispensing system. These specialists test the system to ensure it accurately measures and dispenses medications, records data correctly, and complies with FDA regulations. Their role is crucial in preventing errors and ensuring the system’s reliability and safety for patients.
 5. Clinical Trial Support Staff
5. Clinical Trial Support Staff
As more trials use DHTs like wearables for real-time patient data, skilled staff must ensure data accuracy, integration, and compliance. For example, if a trial uses wearable devices to monitor heart rates, these professionals manage device setup, verify data accuracy, resolve technical issues, and ensure data complies with FDA regulations.
6. Regulatory Submissions and Post-Market Support Staff
DHTs will require accurate classification and regulatory compliance in the desired geographical markets. Experienced professionals will provide guidance or hands-on support of submissions to regulatory bodies for approval of clinical trials or for market clearance and product registration in order to legally market the DHT. These professionals may also support complaint-handling and other post-market activities in accordance with regulatory requirements.
Final Thoughts
The integration of these technologies is an exciting advancement for the life sciences industry. However, it brings with it new challenges in terms of regulation and staffing.
Our team understands the complexities of staffing for life sciences companies, especially when it comes to validation, compliance, and quality assurance. Our full lifecycle staffing services are designed to help you meet the demands of this new era in healthcare. Whether you’re integrating DHT into clinical trials or ensuring your technology complies with FDA standards, Theoris has the staffing expertise to support your organization every step of the way. Learn more!